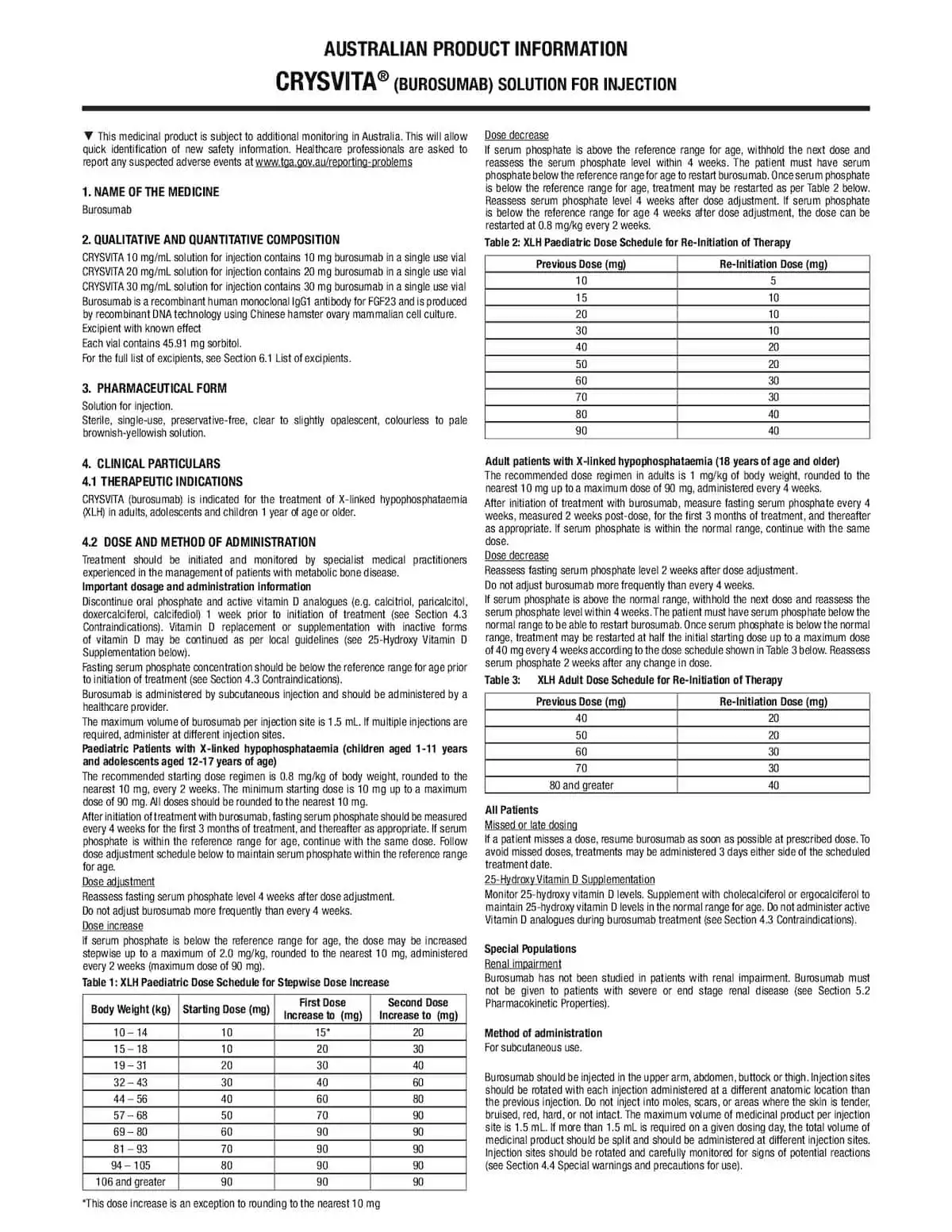
Australian Resources
Downloadable materials
Crysvita® Resources
XLH Resources
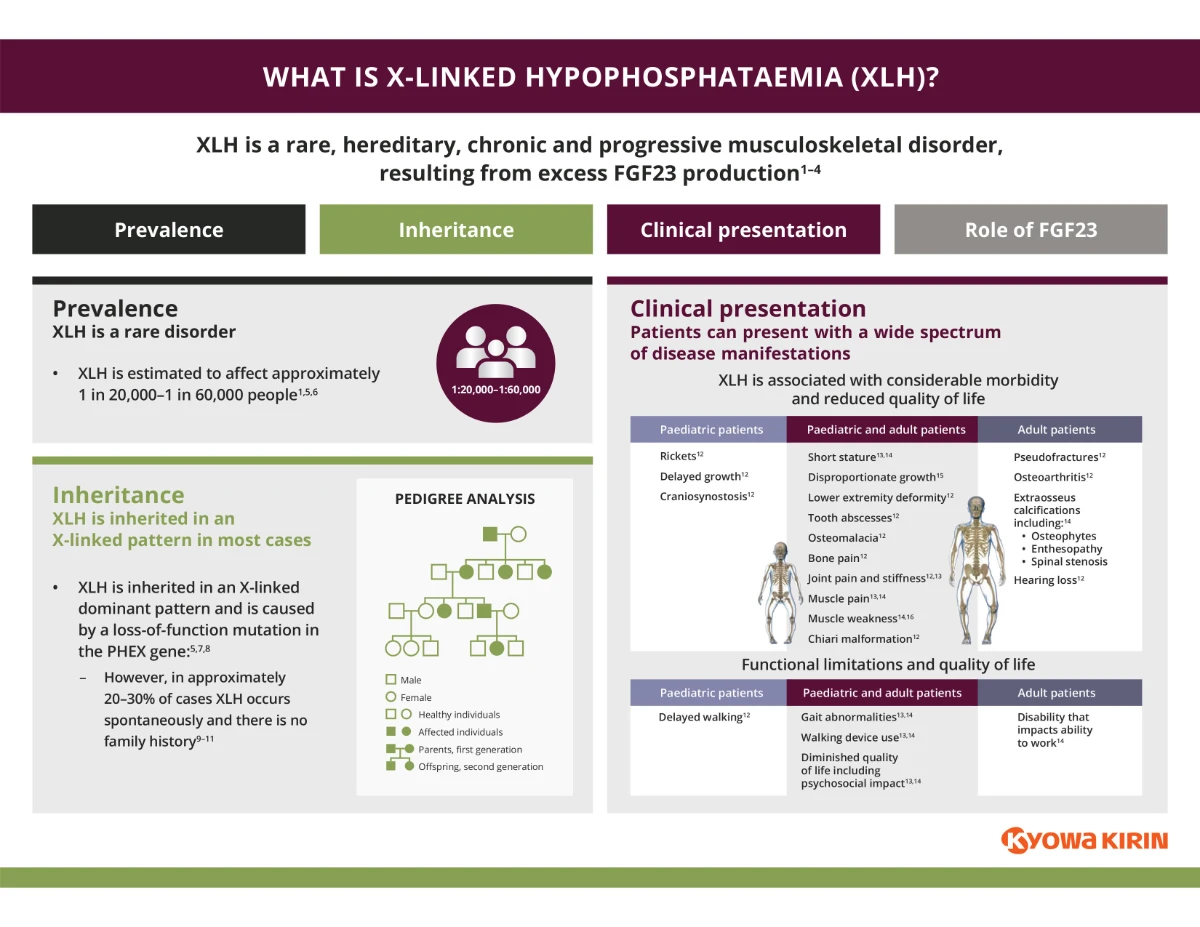
XLH Disease Mechanism
Symposium Highlights
Videos
Podcasts
Management of adult patients with XLH – A/Prof Peter Simm with Dr Penny Coates
Publications of interest
- Portale AA, et al. Continued beneficial effects of burosumab in adults with X-linked hypophosphatemia: Results from a 24-week treatment continuation period after a 24-week double-blind placebo-controlled period. Calcif Tissue Int. 2019;105:271–84.
PubMed link: https://pubmed.ncbi.nlm.nih.gov/31165191/ - Insogna KL, et al. Burosumab improved histomorphometric measures of osteomalacia in adults with X-linked hypophosphatemia: A phase 3, single-arm, international trial. J Bone Miner Res. 2019;34:2183–91
PubMed link: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6916280/ - Haffner D, et al. Clinical practice recommendations for the diagnosis and management of X-linked hypophosphataemia. Nat Rev Nephrol. 2019;15:435-55.
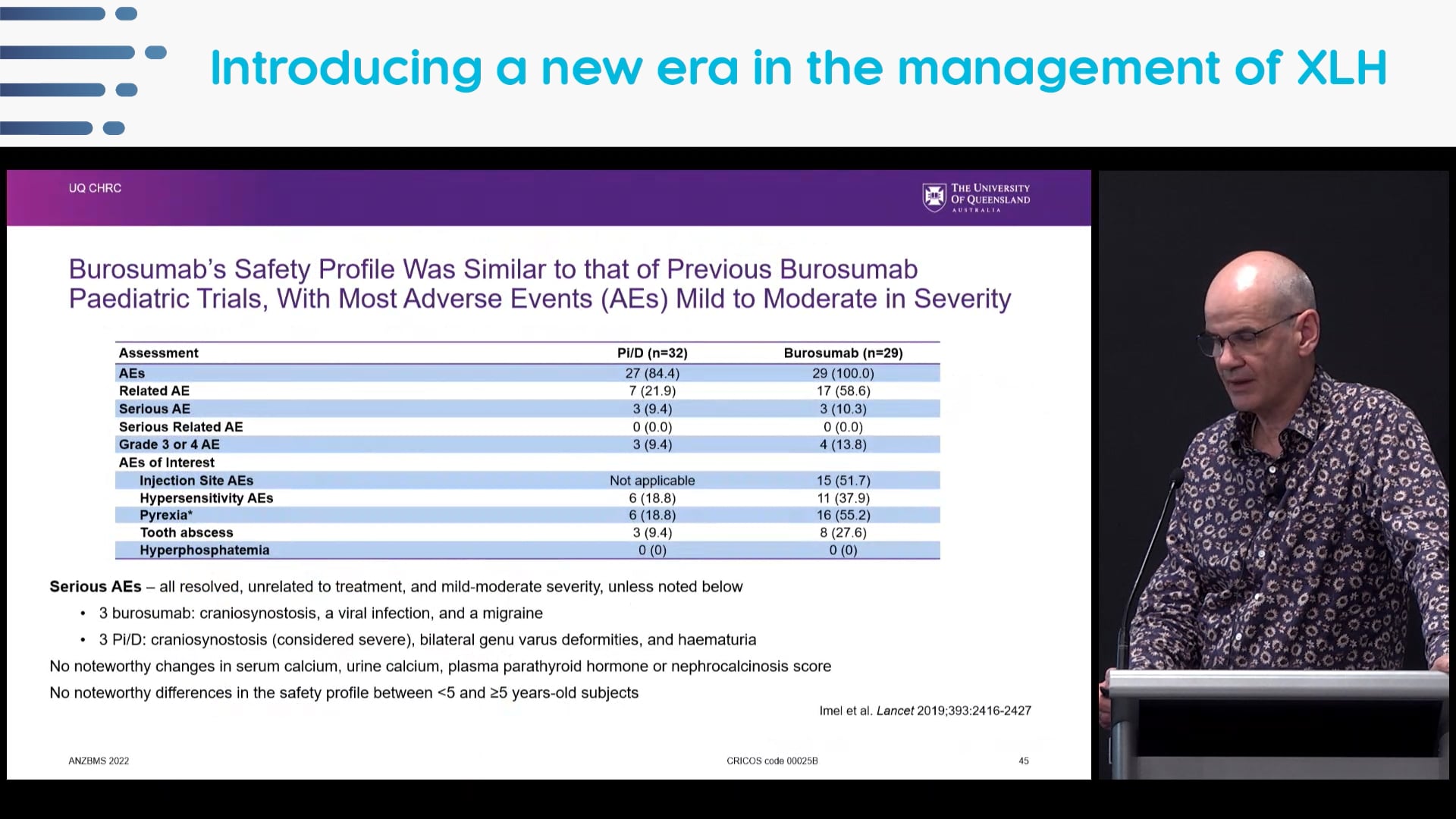
PubMed link: https://www.ncbi.nlm.nih.gov/pubmed/31068690 - Imel EA, et al. Burosumab versus conventional therapy in children with X-linked hypophosphataemia: a randomised, active-controlled, open-label, phase 3 trial. Lancet. 2019;393:2416-27.
PubMed link: https://www.ncbi.nlm.nih.gov/pubmed/31104833 - Whyte MP, et al. Efficacy and safety of burosumab in children aged 1–4 years with X-linked hypophosphataemia: a multicentre, open-label, phase 2 trial. Lancet Diabetes Endocrinol. 2019;7:189-99.
PubMed link: https://www.ncbi.nlm.nih.gov/pubmed/30638856 - Beck-Nielsen SS, et al. FGF23 and its role in X-linked hypophosphatemia-related morbidity. Orphanet J Rare Dis. 2019;14:58.
PubMed link: https://www.ncbi.nlm.nih.gov/pubmed/30808384 - Insogna KL, et al. A randomized, double-blind, placebo-controlled, phase 3 trial evaluating the efficacy of burosumab, an anti-FGF23 antibody, in adults with X-linked hypophosphatemia: Week 24 primary analysis. J Bone Miner Res. 2018;33:1383-93.
PubMed link: https://pubmed.ncbi.nlm.nih.gov/29947083/ - Carpenter TO, et al. Burosumab therapy in children with X-linked hypophosphatemia. N Engl J Med. 2018;378:1987-98.
PubMed link: https://www.ncbi.nlm.nih.gov/pubmed/29791829
Publicly Available Websites
Publicly Available Websites
XLH information websites for healthcare professionals

1. XLHLink.asia (Healthcare professional website)
https://www.xlhlink.asia/hcp/
This website is developed and funded by Kyowa Kirin Asia Pacific Pte. Ltd.

1. The Australian Bone and Mineral Society (ANZBMS)
https://www.anzbms.org.au/

2. The Australasian Paediatric Endocrine Group (ANZSPED)
https://anzsped.org/ /

3. The Australian and New Zealand Society of Nephrology (ANZSN)
https://nephrology.edu.au/
XLH Information websites for patients

1. XLHLink.asia (Patient website)
https://www.xlhlink.com.au/patientstories/
This website is developed and funded by Kyowa Kirin Australia Pty. Ltd.

2. XLH Australia Incorporated
https://xlhaustralia.com/

3. Rare Voices Australia
https://rarevoices.org.au/
We are not affiliated, associated, authorised, endorsed by, or in any way officially connected with any other company, agency or government agency listed hereunder or otherwise.
1. Beck-Nielsen SS, et al. Eur J Endocrinol. 2009;160:491–7. 2. Endo I, et al. Endocr J. 2015;62:811–6. 3. Carpenter TO, et al. J Bone Miner Res. 2011;26:1381–8. 4. Haffner D, et al. Nat Rev Nephrol. 2019;15:435–55. 5. Rafaelsen S, et al. Eur J Endocrinol. 2016;174:125–36. 6. Rajah J, et al. Eur J Pediatr. 2011;170:1089–96. 7. Raimann A, et al. Wien Med Wochenschr. 2020;170:116–23. 8. Razzaque MS. Nat Rev Endocrinol. 2009;5:611–9. 9. Linglart A, et al. Endocr Connect. 2014;3:R13–30. 10. Beck-Nielsen SS, et al. Orphanet J Rare Dis. 2019;14:58. 11. CRYSVITA® (burosumab). Based on Singapore Package Insert. Kyowa Kirin Asia Pacific Pte Ltd; May 2021. 12. Portale AA, et al. Calcif Tissue Int. 2019;105:271–84. 13. Imel EA, et al. Lancet. 2019;393:2416–27. 14. Insogna KL, et al. J Bone Miner Res. 2019;34:2183–91. 15. Carpenter TO, et al. N Eng J Med. 2018;378:1987–98. 16. Insogna KL, et al. J Bone Miner Res. 2018;33:1383–93