Treatment with CRYSVITA® in children with XLH demonstrated significant improvement in phosphate levels, rickets severity and mobility¹⁻³
Table of Contents
Phase II efficacy and safety
Efficacy and safety of CRYSVITA® in children aged 1 to 4 years with XLH has been investigated in a phase II study2
Study design2
An open-label, phase II trial held at 3 sites to investigate the efficacy and safety of CRYSVITA® in 13 young children, aged 1–4 years at baseline, with XLH. The study was 64 weeks and participants could continue to receive CRYSVITA® for up to an additional 96 weeks during the extension period.

†CRYSVITA® dosing: Initiated at 0.8 mg/kg every 2 weeks for 64 weeks. The dose was increased to 1.2 mg/kg if two consecutive pre-dose serum phosphate concentrations were below 1.03 mmol/L, serum phosphate had increased by less than 0.16 mmol/L from baseline and a dose of CRYSVITA® had not been missed.
Co-Primary endpoints2
- Change from baseline in serum phosphate concentration at week 40
- Safety
Secondary endpoints2
- Change from baseline in RGI-C at week 40 and week 64
- Pharmacodynamics: Change from baseline in ALP and serum 1,25(OH)2D
- Change in recumbent length (or standing height Z scores), physical ability (evaluated by the 6MWT) and patient reported pain and functional disability
Key findings2
- CRYSVITA® increased fasting serum phosphate concentrations to within the normal range and were sustained throughout the study
- CRYSVITA® resulted in the substantial healing of rickets and a positive lower limb deformity score by week 40 in all patients, indicating improvement
- Gains were sustained at week 64 and were consistent with improvements seen with CRYSVITA® in children aged 5–12 years in another Phase II study
- CRYSVITA® increased serum 1,25(OH)2D
- CRYSVITA® had an acceptable safety profile
CRYSVITA® treatment starting at 0.8 mg/kg in children with XLH aged 1–4 years restores phosphate homeostasis and significantly improves rickets2
Phase III efficacy and safety
Efficacy and safety of CRYSVITA® in children with XLH has been investigated in a phase III study1
Study design1
A randomised, open-label, parallel-group, phase II trial at 9 sites, to investigate the efficacy and safety of CRYSVITA® in 52 children, aged 5–12 years, with XLH. The study was 64 weeks and patients had the option to enrol in an open-label extension.

†CRYSVITA® dosing: Initiated at 0.1 mg/kg every 2 weeks or 0.2 mg/kg every 4 weeks. If no severe side-effects were observed, patients were assigned sequentially to receive escalating doses of 0.2 or 0.3 mg/kg every 2 weeks, or 0.4 or 0.6 mg/kg every 4 weeks.
Primary endpoints1
- Change from baseline in total RSS at week 40 and week 64
Secondary endpoints1
- Change from baseline in RGI-C at week 40 and week 64
- Pharmacodynamics: Change from baseline in serum phosphate, TmP/GFR, serum 1,25(OH)2D, ALP
- Change in growth velocity and height Z scores
- Safety
Key findings1
- CRYSVITA® treatment increased serum phosphate levels to within normal limits
- CRYSVITA® administered once every 2 weeks provided a sustained increase in the serum phosphate level to normal or near normal levels after dose adjustment
- Every 4-week dosing was associated with lower levels of serum phosphate at the end of each dose interval
- The improvement in phosphate metabolism corresponded to a decrease in rickets severity
- This may have contributed to concurrent improvements in growth and physical activity and a reduction in pain
- CRYSVITA® treatment improved RGI-C scores, with a small but significant reduction in leg deformity
- There were no adverse events leading to study discontinuation, treatment discontinuation or death
- All other TEAEs were mild or moderate
CRYSVITA® treatment provides therapeutic benefits by restoring phosphate homeostasis and improving rickets in children with XLH aged 5—12 years, who previously received conventional therapy with limited clinical improvement1
Following the positive findings from the phase II trials, a phase III clinical study was conducted to investigate the efficacy and safety of CRYSVITA® compared with conventional therapy in children aged 1—12 years3.
Efficacy and safety of CRYSVITA® in children with XLH has been investigated in a phase III study3
Study design3
A randomised, active-controlled, open-label, phase III trial at 16 clinical sites, compared the efficacy and safety of CRYSVITA® with conventional therapy in children with XLH aged 1—12 years, over a 64-week period.3
| Study population | |
|---|---|
| Children with XLH, aged 1—12 years old | N=61 |
| Fasting serum phosphate <0.97 mmol/L (3.0 mg/dL) | Confirmed PHEX mutation or variant of unknown significance in the patient or a family member |
Prior conventional therapy
| Total RSS ≥2.0 |

†CRYSVITA® dosing: Initiated at a dose of SC 0.8 mg/kg Q2W; increased to 1.2 mg/kg Q2W if two consecutive pre-dose, fasting, serum phosphate concentrations were below 1.03 mmol/L (3.2 mg/dL) and serum phosphate had increased by <0.16 mmol/L (<0.5 mg/dL) from baseline on a single measurement.
‡Conventional therapy dosing: The recommended oral phosphate dose in children is 20–60 mg/kg/day divided into three to five doses per day and alfacalcidol 40–60 ng/kg/day or calcitriol 20–30 ng/kg/day; depending on the formulation, the active vitamin D could be given one to three times a day.
§Primary assessments of recumbent length/standing height Z score and lower limb deformity score are at Week 64.
Primary endpoint3
- Change in rickets severity from baseline at Week 40 as assessed by the RGI-C global score
Secondary endpoints3
- Change in rickets severity from baseline at Week 64 as assessed by the RGI-C global score
- Proportion of subjects with a mean RGI-C global score ≥ +2.0 (substantial healing) at Week 40 and 64
- Change from baseline at Week 40 and 64 in Thacher RSS
- Change from baseline in lower limb deformity as assessed by RGI-C long leg score at Week 40 and 64
- Change from baseline at Week 40 and 64 in growth outcomes:
- Height-for-age Z scores
- Growth velocity
- Change from baseline in the 6MWT total distance and percent of predicted normal (for subjects ≥ 5 years of age at the screening visit) at Weeks 24, 40, and 64
- Change from baseline over time in serum phosphate
- Change from baseline over time in serum 1,25(OH)2D
- Change from baseline over time in ratio of TmP/GFR
- Change from baseline over time in ALP
Patient characteristics3
| Characteristic | Conventional therapy (n=32) | CRYSVITA® SC Q2W (n=29) |
|---|---|---|
| Age, years, mean (SD) | 6.3 (3.2) | 5.8 (3.4) |
| Girls, n (%) | 18 (56%) | 16 (55%) |
| Boys, n (%) | 14 (44%) | 13 (45%) |
| White, n (%) | 25 (78.1%) | 25 (86.2%) |
| Height Z score, Mean (SD)Median [Range] | –2.1 (0.9) –2.1 [–2.51 to –1.44] | –2.3 (1.2) –2.3 [–3.05 to –1.45] |
| Weight Z score, Mean (SD)Median [Range] | –0.6 (0.9) –0.7 [-1.17 to 0.05] | –0.9 (1.2) –0.8 [-1.75 to 0.59] |
| Tanner stage, n(%) | ||
| 1 | 31 (97%) | 27 (93%) |
| 2 | 1 (3%) | 2 (7%) |
| Serum phosphate, mmol/L, mean (SD) | 0.74 (0.08) | 0.78 (0.08) |
| Serum TmP/GFR, mmol/L, mean (SD) | 0.65 (0.11) | 0.71 (0.12) |
| Serum 1,25(OH)2D, pmol/L, mean (SD) | 96 (36) | 110 (48) |
| Serum 25(OH)D, nmol/L, mean (SD) | 79.38 (25.14) | 80.63 (26.15) |
| ALP, U/L, mean (SD) | 523.4 (154.4) | 510.8 (124.9) |
| Duration of conventional therapy, years, mean (SD) Median [min, max] |
4.3 (3.0) 3.5 [1.88, 6.33] |
3.3 (3.1) 2.2 [1.56, 3.47] |
| Total Thacher RSS, mean (SD) Median [range] |
3.2 (1.1) 3.0 [2.50, 4.00] |
3.2 (1.0) 3.0 [2.50, 3.50] |
Baseline values were assessed after a 7-day wash-out period, in which patients stopped treatment with conventional therapy.
Phosphate regulation
Treatment with CRYSVITA® restores phosphate homeostasis in children with XLH3
CRYSVITA® achieved significantly greater improvements in mean serum phosphate levels compared with conventional therapy as early as Week 2, which were sustained over 64 weeks3
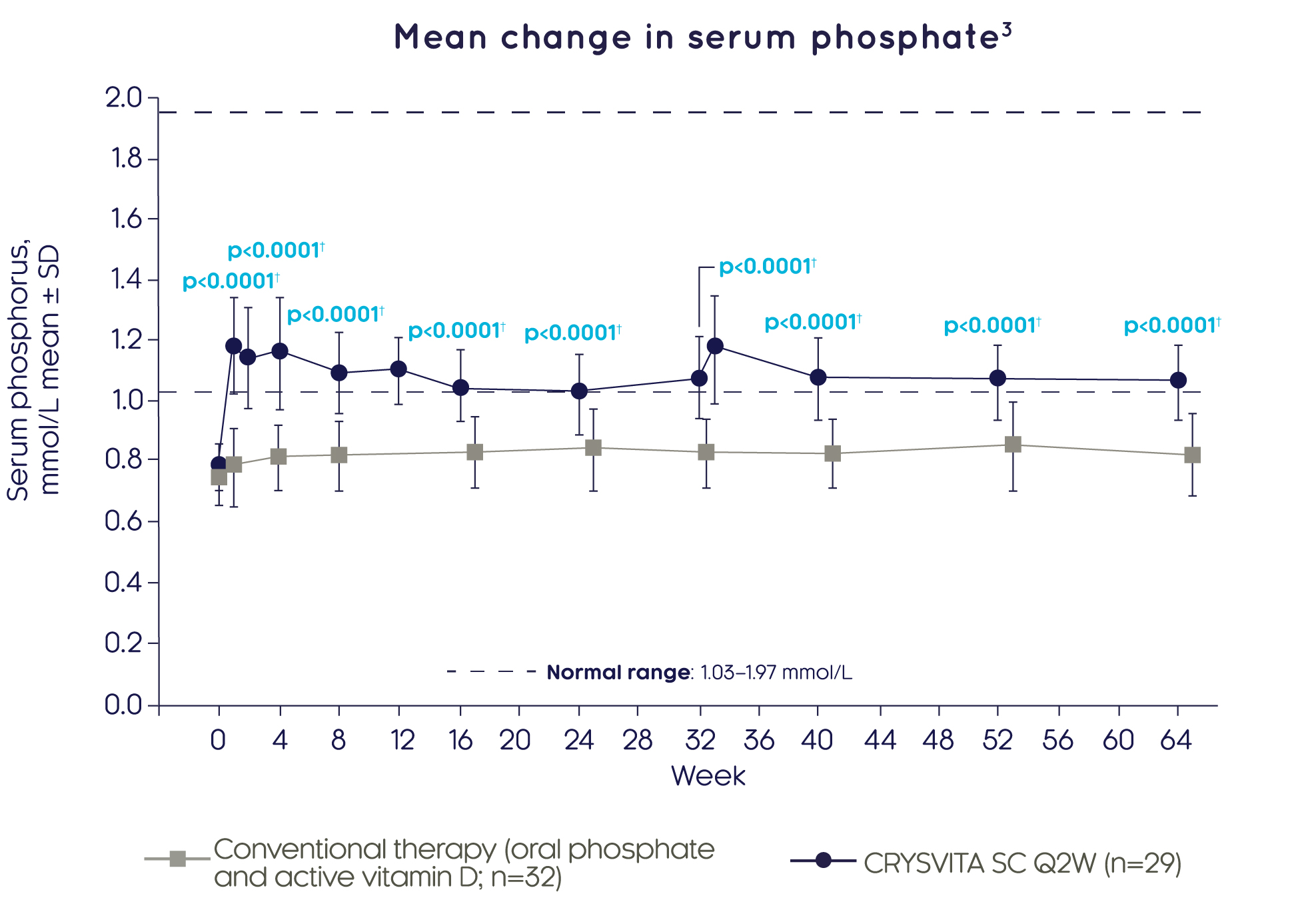
Adapted from Imel EA, et al. 20193
†Based on comparison between treatment groups in the LS mean change from baseline using a GEE model.
Assessments at Weeks 2, 12 and 33 only occurred in the CRYSVITA® group. Post baseline values are off set to avoid overlapping error bars.
CRYSVITA® achieved significantly greater improvements in renal phosphate reabsorption (as measured by TmP/GFR) compared with conventional therapy as early as Week 4, which were sustained over 64 weeks3
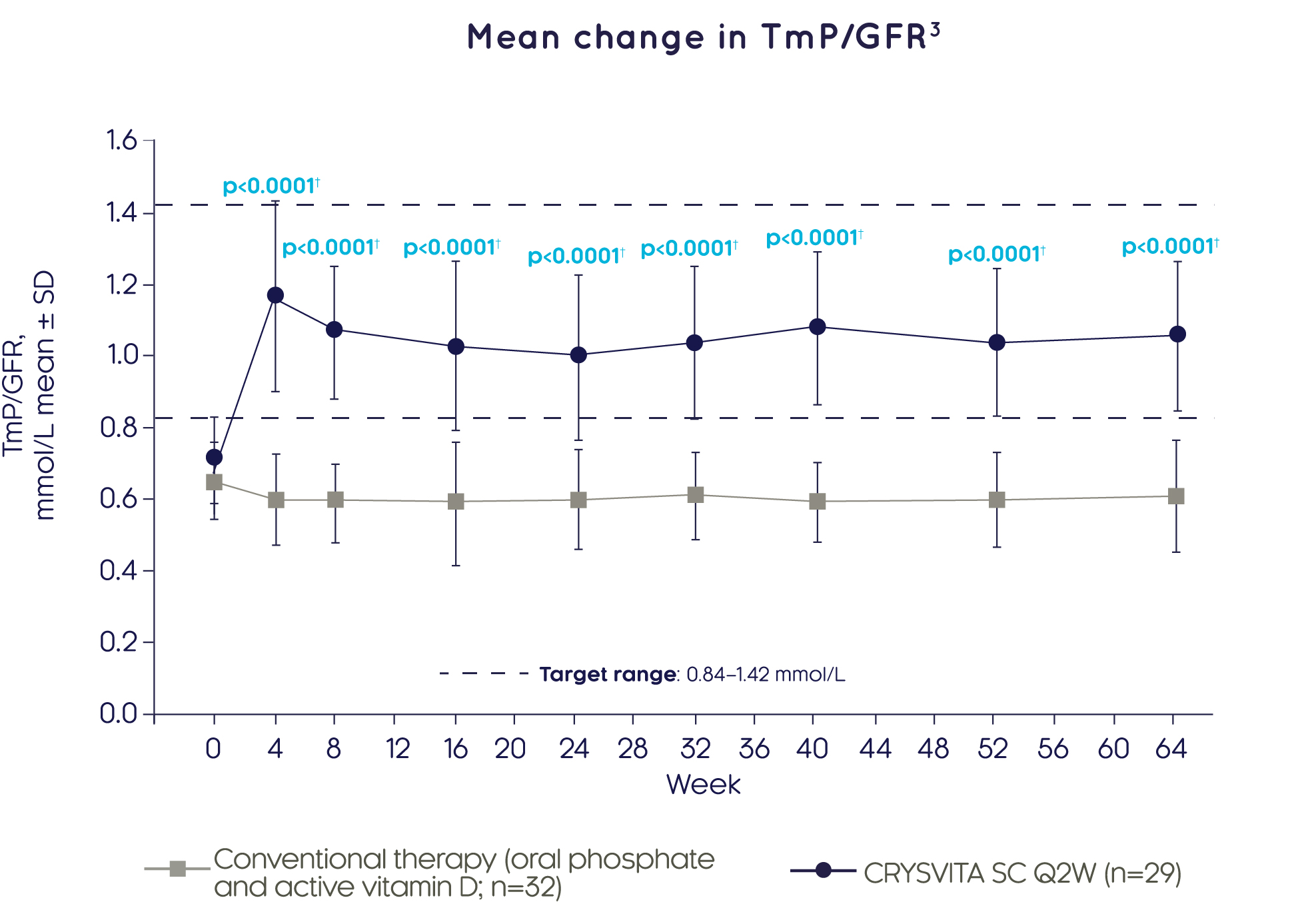
Adapted from Imel EA, et al. 20193
†Based on comparison between treatment groups in the LS mean change from baseline using a GEE model.
Bone health
CRYSVITA® improves biochemical markers of bone health3
CRYSVITA® achieved significantly greater reductions in ALP activity compared with conventional therapy over 64 weeks3

Adapted from Imel EA, et al. 20193
†Based on comparison between treatment groups in the LS mean change from baseline using a GEE model. Some post-baseline values are slightly offset from the actual treatment week to avoid overlapping error bars.
‡Normal range varies depending on sex and age within males and females aged 1–15 years; the upper limit ranged from 297 to 385 U/L.
Rickets
CRYSVITA® heals and reduces severity of rickets in children with XLH3
CRYSVITA® achieved significantly higher RGI-C global scores versus conventional therapy at Week 40 and Week 643

Adapted from Imel EA, et al. 20193
†Based on the comparison between treatment groups in the LS mean change, using the ANCOVA model at Week 40 and the GEE model for Week 64.
CRYSVITA® achieved substantial or complete healing of rickets (RGI-C score ≥2.0) in a significantly greater proportion of children (87%) than conventional therapy (19%) at Week 643

†GEE model includes treatment, visit, interaction between treatment group and visit and baseline age stratification as factors, and baseline RSS total score as a continuous covariate.
CRYSVITA® achieved significantly greater improvements in total Thacher RSS compared with conventional therapy at Week 40 and Week 643
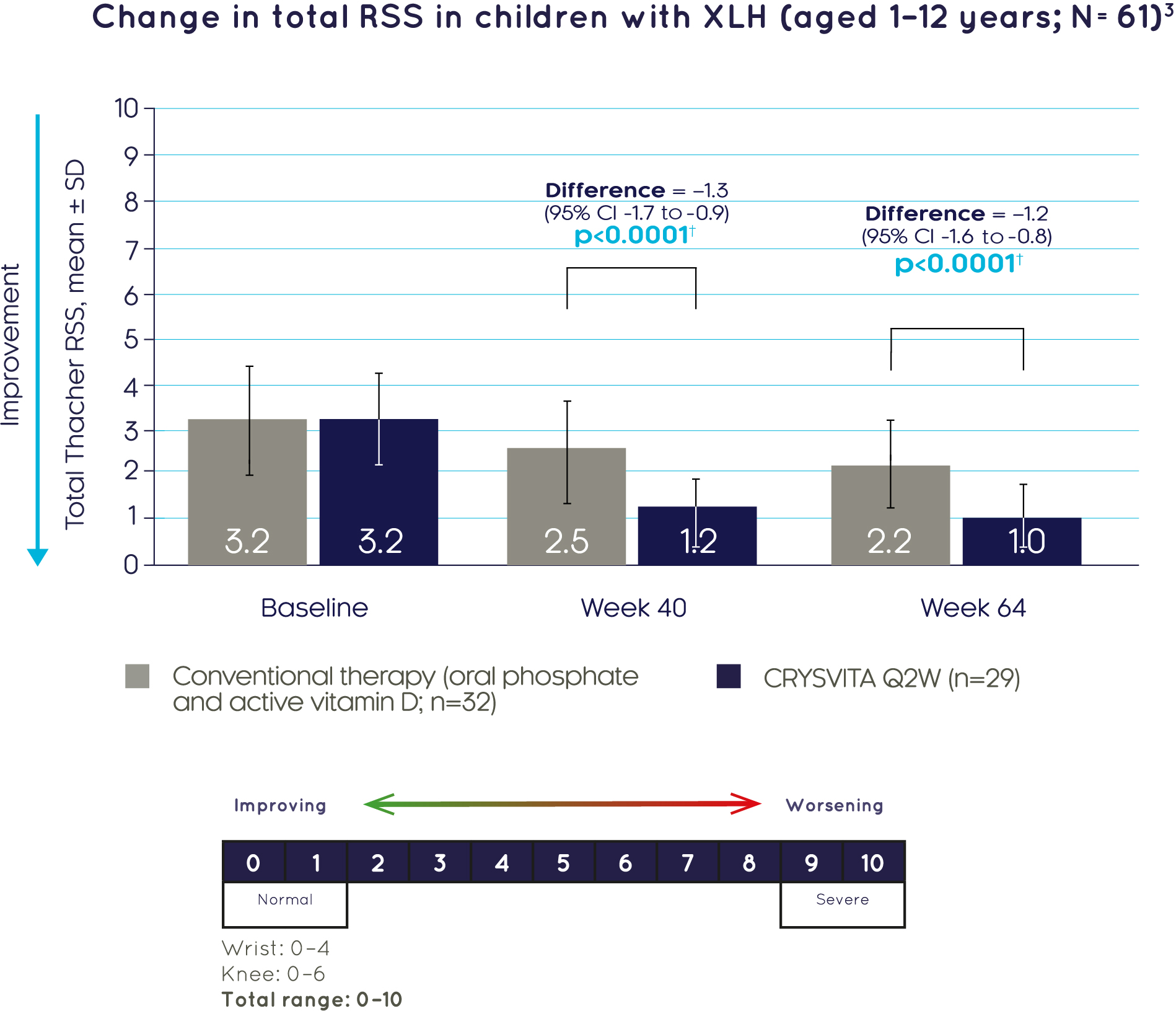
†Based on the comparison between treatment groups in the LS mean change, using the ANCOVA model at Week 40 and the GEE model for Week 64.
Improvement in rickets in a 4-year-old girl with XLH treated with CRYSVITA®3

| Score | Baseline | Week 40 |
|---|---|---|
| Thacher RSS | ||
| Wrist | 2.0 | 0.5 |
| Knee | 1.5 | 1.0 |
| Total | 3.5 | 1.5 |
| RGI-C score† | ||
| Wrist | - | +2.3 |
| Knee | - | +2.0 |
| Global | - | +2.0 |
†Substantial healing is defined as an RGI-C score ≥+2.0.
Lower limb deformity
CRYSVITA® helps correct lower leg deformity in children with XLH3
CRYSVITA® achieved significantly higher RGI-C lower limb deformity scores versus conventional therapy at Week 40 and Week 643

Adapted from Imel EA, et al. 20193
†Based on the comparison between treatment groups in the LS mean change from baseline using the GEE model.
Growth
CRYSVITA® prevents early decline in growth in children with XLH3
CRYSVITA® achieved significantly greater improvements in recumbent length/standing height Z score compared with conventional therapy at Week 40 and Week 643

Adapted from Imel EA, et al. 20193
†Based on comparison between treatment groups in the LS mean change from baseline using the GEE model for recumbent length/standing height Z score and the ANCOVA model for growth velocity Z score. Some post-baseline values are slightly offset from the actual treatment week to avoid overlapping error bars.
CRYSVITA® achieved significantly greater improvements in mean growth velocity Z score versus conventional therapy at Week 643

†Based on comparison between treatment groups in the LS mean change from baseline for recumbent length/standing height Z score and the ANCOVA model for growth velocity Z score.
Mobility
CRYSVITA® improves mobility in children with XLH3
CRYSVITA® achieved significantly greater increases in percent predicted distance walked in the 6MWT compared with conventional therapy at Week 643
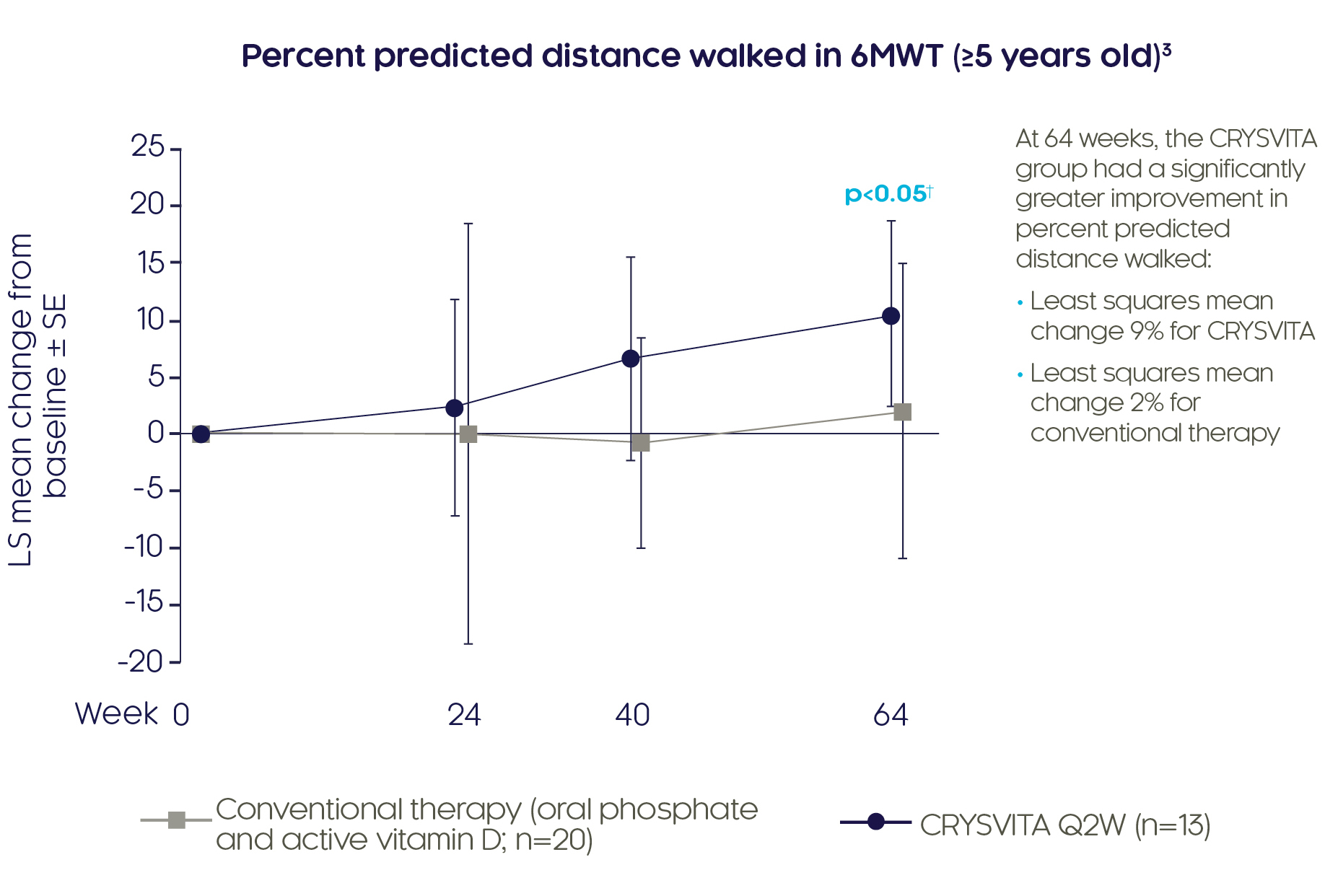
Adapted from Imel EA, et al. 20193
†Based on comparison between treatment groups in the LS mean change from baseline using a GEE model.
6MWT was assessed in patients ≥5 years old and able to complete the test. Some post-baseline values are slightly offset from the actual treatment week to avoid overlapping error bars.
Safety
Most common treatment-emergent adverse events (TEAEs)† reported in children with XLH up to 64 weeks3
| Assessment | Conventional therapy (n=32) n (%) | CRYSVITA® SC Q2W (n=29) n (%) |
|---|---|---|
| Pyrexia | 6 (19%) | 16 (55%) |
| Cough | 6 (19%) | 15 (52%) |
| Arthralgia | 10 (31%) | 13 (45%) |
| Vomiting | 8 (25%) | 12 (41%) |
| Nasopharyngitis | 14 (44%) | 11 (38%) |
| Pain in extremity | 10 (31%) | 11 (38%) |
| Headache | 6 (19%) | 10 (34%) |
| Injection site erythema | 0 | 9 (31%) |
| Dental caries | 2 (6%) | 9 (31%) |
| Tooth abscess | 3 (9%) | 8 (28%) |
| Injection site reaction‡ | 0 | 7 (24%) |
| Rhinorrhoea | 2 (6%) | 7 (24%) |
| Diarrhoea | 2 (6%) | 7 (24%) |
| Vitamin D decrease | 1 (3%) | 6 (21%) |
| Constipation | 0 | 5 (17%) |
†Adverse reactions in the frequency category of ‘very common’ (≥1/10); ‡Injection site reaction is a grouped term that includes injection site reaction, erythema, pruritus, rash, erosion, swelling, urticaria, discomfort, hypersensitivity, inflammation and papule. Please refer to the full Prescribing Information and publication3 for details on full safety profile of CRYSVITA®.
1. Carpenter TO, et al. N Engl J Med. 2018;378:1987–98. 2. Whyte MP, et al. Lancet Diabetes Endocrinol. 2019;7:189–99. 3. Imel EA, et al. Lancet. 2019;393:2416–27.






